
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
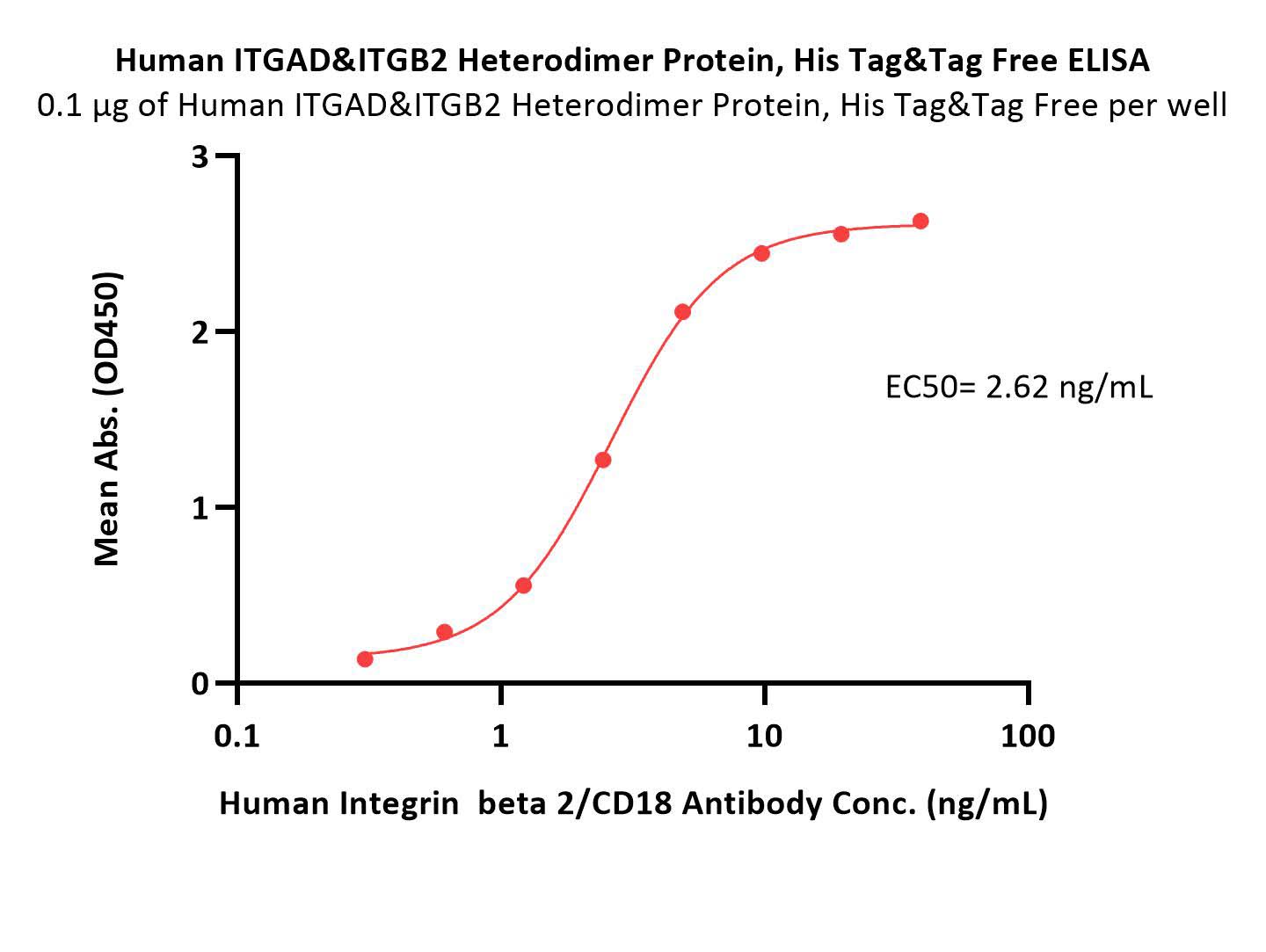
| IT2-H53W6 | Human | Human Integrin alpha D beta 2 (ITGAD&ITGB2) Heterodimer Protein, His Tag&Tag Free |  |

|
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Natalizumab biosimilar(Polpharma Biologics) | PB-006; DST-356A1 | Approved | Polpharma Biologics Sa | Tyruko | United States | Multiple Sclerosis; Crohn Disease | Sandoz Inc | 2023-08-24 | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Crohn Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ATL-1102 | ATL-1102; ATL/TV-1102; ISIS-107248; TV-1102 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Multiple Sclerosis; Muscular Dystrophy, Duchenne | Details |
| BIIB-107 (Biogen) | BIIB-107 | Phase 1 Clinical | Biogen Inc | Multiple Sclerosis | Details |
| ELND-004 | ELND-004 | Phase 1 Clinical | Perrigo Company Plc | Autoimmune Diseases | Details |
This web search service is supported by Google Inc.







